Clinical-Grade IBD Monitoring.
No Portal Required.
Your patients arrive with 60 days of objective vitals and validated symptom scores. You start the appointment informed.
The Gap Between Visits
Patients forget what happened. Flares are reported after the fact. You spend the first 10 minutes of every appointment reconstructing history from imperfect recall.
Paper diaries are incomplete. Portal questionnaires have 20% completion rates. You're making clinical decisions with incomplete data.
What Your Patients Bring You
Patients generate comprehensive PDF reports in 30 seconds. No training required.
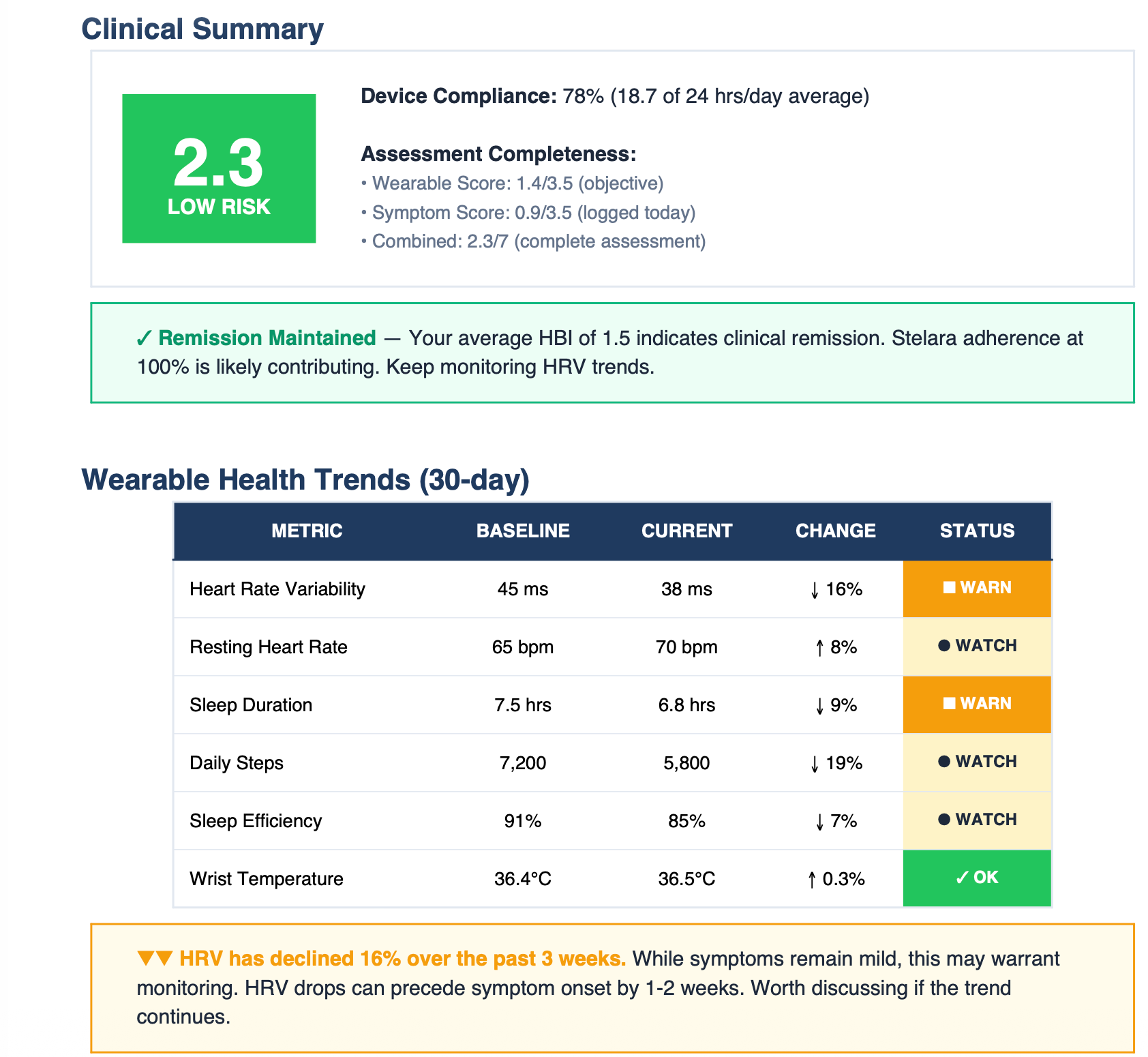
Clinical Summary
Risk score (0-7), device compliance, remission status at a glance.
Wearable Trends
HRV, resting heart rate, sleep objective data you can't get from recall.
Flare Timeline
When flares happened, duration, severity, pre-flare patterns.
Medication Adherence
Actual percentages, not self-reported guesses.
HBI/SCCAI Scores
Validated clinical indices tracked continuously.
Questions for Provider
Auto-generated based on patient data patterns.
The Methodology You Trust Now Continuous
Flarity combines objective wearable data with subjective symptom input.
Wearable Layer (0-3.5)
Apple Watch monitors HRV, heart rate, sleep, temperature against personal baseline. Detects physiological changes before symptoms appear.
Symptom Layer (0-3.5)
When signals deviate, patients complete a 30-second check-in based on HBI (Crohn's) or SCCAI (UC). No survey fatigue.
Combined Score (0-7)
Objective + subjective = the same methodology you use. Biomarkers plus clinical assessment.
Wearables alone can't assess pain or urgency. Symptoms alone rely on imperfect memory. Combined assessment like labs plus exam gives the complete picture.
Built on Peer-Reviewed Science
Based on a 2025 Mount Sinai study of 300+ IBD patients, published in Gastroenterology the leading peer-reviewed journal in gastroenterology.
The research demonstrated that wearable signals can detect flare patterns up to 7 weeks before symptoms appear. Flarity implements a conservative 3-7 day prediction window for actionable, real-world accuracy.
Zero Burden for Your Practice
No Portal
Patients share reports via email, AirDrop, or patient portal upload. Nothing to install.
No Training
Patients generate reports in 30 seconds. No onboarding required.
No Logins
You receive clinically formatted PDFs. Works with your existing workflow.
EHR integration (FHIR, Epic MyChart) on our roadmap. Interested? Let's talk.
Speaks Your Language
The validated scoring systems you trust, tracked continuously.
Harvey-Bradshaw Index (HBI)
Crohn's disease
SCCAI
Ulcerative colitis
Montreal Classification
Every report
Easy for Patients. Valuable for You.
No daily logging. The Watch monitors passively. Smart prompts only when their body shows changes. 30-second check-ins, free 21-day trial.
Built by Someone Who Needed It
Flarity was created by an engineer living with Crohn's disease someone who's been through the ER visits, the scope preps, the uncertainty between appointments.
After an ileocecal resection, he built the tool he wished existed: continuous monitoring that speaks his gastroenterologist's language.
Flaresense Inc. is based in Toronto, Canada.
Common Questions
Patients generate PDF reports in the app with one tap. They can share via email, AirDrop, or upload to your patient portal. No integration required on your end.
Flarity is an iOS app (iPhone required). For wearables, it works with Apple Watch (best experience), Oura Ring, Whoop, Garmin, and Fitbit, all via Apple Health sync. Patients without a wearable can still use iPhone-only mode with symptom tracking and basic activity data.
Harvey-Bradshaw Index (HBI) for Crohn's disease, Simple Clinical Colitis Activity Index (SCCAI) for ulcerative colitis, and Montreal Classification for disease location/behavior. The same validated tools you use.
Flarity is a wellness application, not an FDA-cleared medical device. It's designed to support patient self-tracking and clinician communication, not to diagnose or treat.
Not yet, but EHR integration (FHIR, Epic MyChart) is on our roadmap. Currently, patients share reports directly with you. Interested in early integration access? Contact us.
Patients can try Flarity free for 21 days. After that, plans are $14.99/month or $149/year. There's no cost to you or your practice.
The 2025 Mount Sinai study detected flare signals up to 7 weeks early under research conditions. Flarity conservatively targets up to 4 weeks advance warning to account for real-world variability and ensure clinically actionable accuracy. Prediction improves as the app learns each patient's personal baseline over time.
Get in Touch
Questions about recommending Flarity? Interested in a pilot program or EHR integration? We'd love to hear from you.
Or email us directly:
support@getflarity.com